Vapor Finishing for Printed Parts
BACKGROUND
Vapor finishing uses acetone to smooth finish 3D prints by filling the micropores present in the plastic that result from printing. The process of vapor finishing saturates the air around the part to make the surface “flow” into a smoother, better finish. There are two types of acetone bath; cold finishing and hot finishing, both use pure acetone to vapor finish the printed part. The benefits of cold baths being that the part can be left in the bath for multiple hours, and the fumes are more easily controlled. Boiling acetone (temperature of 132 degrees Fahrenheit) is a faster method than cold finishing. Results of boiling acetone can be seen in a matter of seconds, often outweighing the dangers of boiling the chemical [SOURCE]. By smoothing out a printed part the stress concentrations that are created when the part is printed, are decreased. This is done by decreasing the print lines and the micro-pores and micro-cracks in the plastic by essentially filling these in with acetone [SOURCE]. For both cool and hot vapor finishing, parts require a cooling time that ensures all the acetone has been evaporated and that the part has completely hardened. To make sure of this, pieces could use 12-18 hours before being implemented in designs. This will also make sure no warping or pooling of the acetone has occurred between ridges on the part.
DESIGN:
The vapor finishing design for this experiment included a rice cooker chamber, acetone, 3D printed parts, and scale. This is the easiest method of boiling acetone. For each 3D cap, the time left in the bath, the temperature, and the time allowed to cool was kept constant. The part was placed in the chamber, the specific amount of acetone was added, and then the heat was turned. The heat was kept on for the same time for each bath, before being turned off and the lid to the chamber was removed. The part was then massed and used. For each experiment, the heat was turned on for 45 seconds to allow the acetone to completely vaporize. The lid was then removed when one minute had passed. Each part cooled for 15 minutes before being tested. The cooling period was implemented to make sure that excess acetone was evaporated, and the part was hardened.
Summary of Test one:
A total of 10 trials were conducted with acetone levels ranging from 0mL to 19.1mL of acetone. It was found that when a cap was treated with 0mL of acetone there was a percent mass gain of 31.3% while a cap treated with 19mL has a percent mass gain of 16.6%. Mass gain was measured by weighing the caps prior to finishing, letting them cool after finishing, and then screwing them on a bag and squeezing the bag while it is upside down. This meant water was absorbed into the micropores when water was forced into the plastic. Caps in between these two points included 5.3mL with a mass gain of 31%, 10.8mL with mass gain of 19.7%, 14.1mL with mass gain of 21.4%, 16.3 mL with mass gain of 18.5%, and 17.8 mL with mass gain of 18.5%. The other trials were repetitions of these levels with similar results. Other data take in experiment one was the water lost from the bag when the caps were being tested. The initial amount of water was measured in mL. After the bag was tilted and squeezed, the bag was re-massed to see how much water escaped. It was discovered that the more acetone used, the less water that escaped. The untreated cap allowed almost all the water to escape. The cap treated with 19mL only allowed 1.7mL to escape out of 140mL.
Summary of Experiment 2:
This experiment was performed to confirm the results from experiment one. The focus was on one low level acetone level and then many higher-level acetone levels. Since the first experiment established the necessity of the higher levels of acetone, the second experiment was performed to finalize this conclusion. For comparison, an untreated cap was tested with the bag and, again, it allowed lots of water to escape with some pressure, further supporting the need for vapor finishing. Experiment two did support the first one. An additional five trials were completed. One with no treated and four more, all above 17mL. The average mass gain between these four treated caps was 22.7%. These four caps allowed virtually no water to escape when attached to a bag and squeezed upside down, further supporting the conclusion made from experiment one. The untreated cap allowed almost all the water to escape again. The recommended amount of acetone was concluded to be 14-19mL.
Miscellaneous:
Weight of dry cap average: 5.18 g
Weight of cup used to measure acetone: 19.2g
Mass of bag: 11.4 g
Time to dry for each trial: 15 minutes


The photo above compares and untreated cap (top) with the treated caps. There are few noticeable differences, besides the greater amount of rounding in the 19mL level cap (top left).
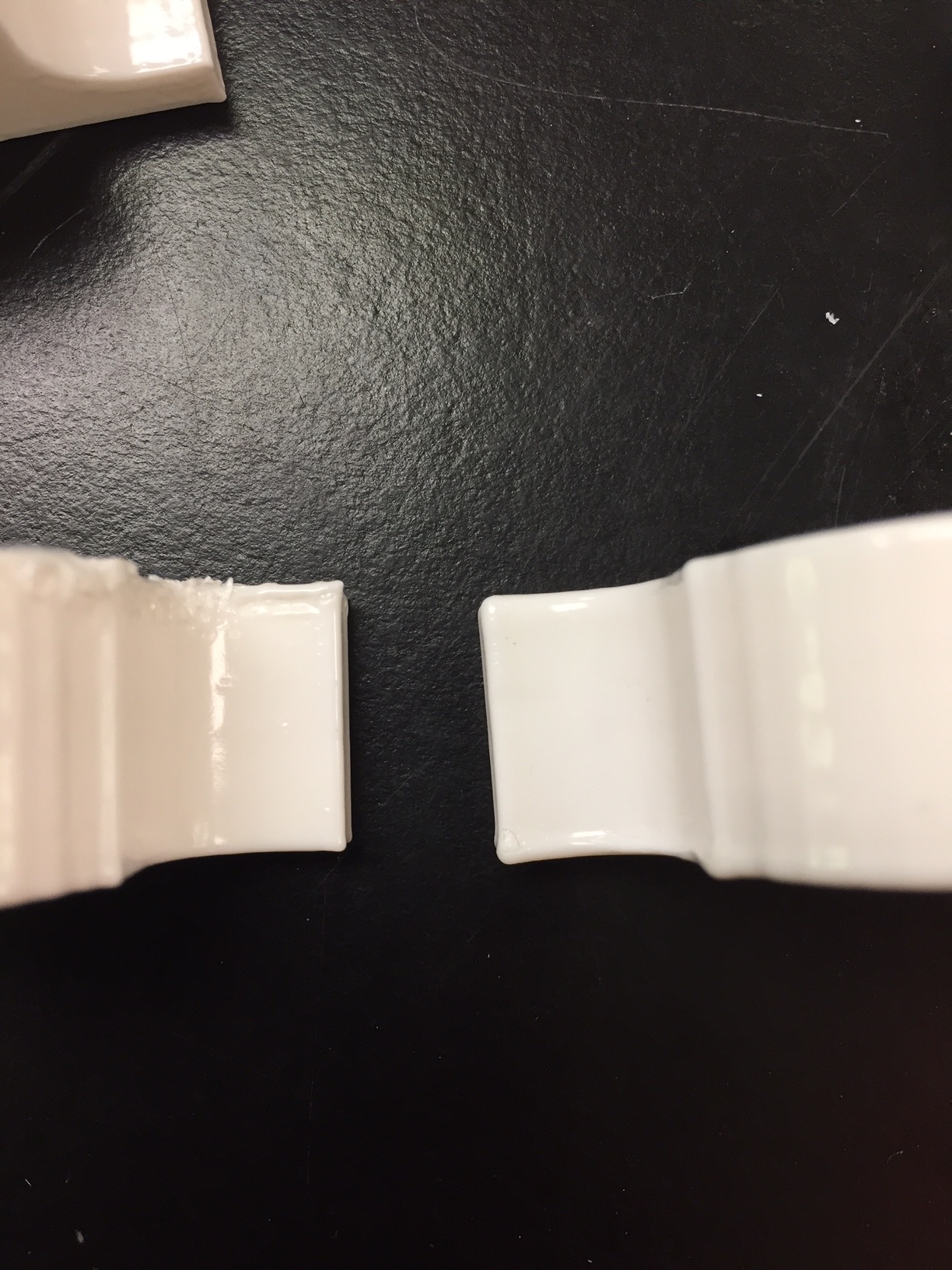

The above picture shows a cap treated with 10mL (L) and a 19 mL (R). The 10mL treated cap is less smooth than the 19mL treated cap. The edges are less rounded. The necessity of more acetone was also supported when the 19mL treated cap allowed less water to both escape the bag and cap connection and infiltrate the cap.
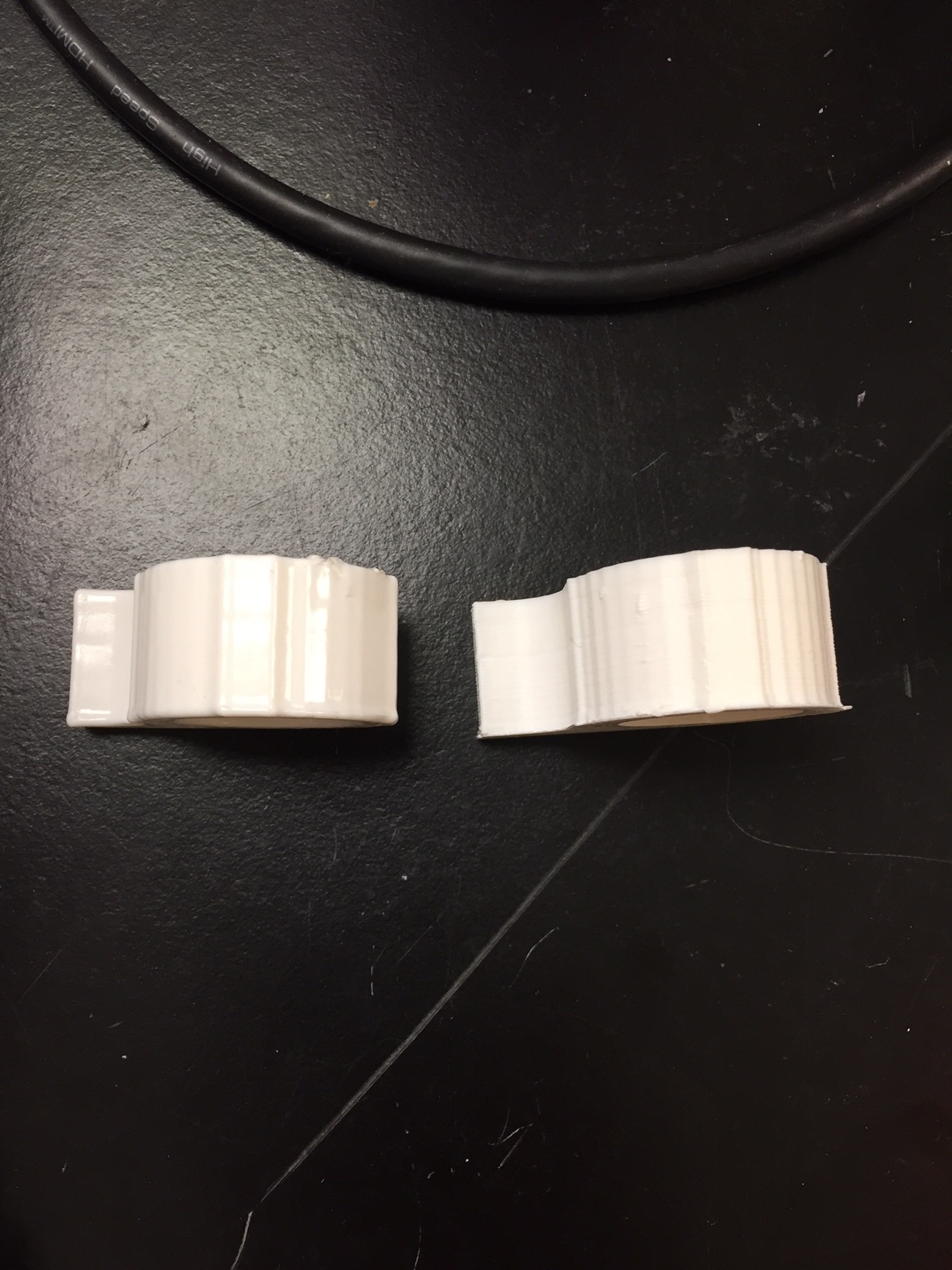

The picture above shows a cap finished with 19mL of acetone (L) and an unfinished cap (R).
The treated cap is much shinier and smoother than the untreated. There are decreased print lines and the texture is more uniform than the untreated.
CONCLUSION
Without any vapor finish, cap and bag connection allowed water to easily escape from the connection point with basic pressure. The largest increase of mass was also associated to the cap without any vapor finish, indicating that water was easily infiltrating the plastic. For the second experiment, the untreated cap did not have a large mass increase, but still allowed water to run out easily.
Increasing the amount of acetone used decreased the amount of water allowed to escape while also increasing the efficiency of the connection. Almost no water escaped at the connection point with even greater pressure compared the pressure applied to the little to no acetone experiments. This was also demonstrated simply by allowing the bag to tilt upside down and seeing that the parts treated with more acetone had less water escaping than untreated parts. The recommended range for small to medium parts is 15-18 mL of acetone. With that in mind, it was also found that levels of 19mL and 17mL both performed well under applied pressure but the 17mL acetone levels kept the exactness of the design better. Meaning the acetone did not smooth the part on the outside as much but performed just as well, and the differences were not very noticeable.
The mass of the caps was measured before and after each trial. To give an idea of how much water was being absorbed during each trial. For each cap treated, when attached to the bag, pressure was applied to test how this affects the amount of water escaping at the bag and cap connection. After pressure was applied and water was allowed run out. It was found that past 17 ml of acetone far less water escaped at the connection. The percent mass gained by the caps at this point was also less. This indicates that the increase in acetone levels helps decrease the amount of water soaking into the part. The greater the acetone levels, the better the webbing formed between the plastic layers. Increasing the acetone that is vaporized increases the acetone that is being deposited between particles in the plastic. This is also why a drying period is necessary to make sure the part has hardened sufficiently before use.
The amount water present after the bag has been squeezed was measured as well. This accounted for any water that escaped the cap through the pores during this process. It was observed that even though the caps still absorbed water with later runs, it was a small amount and most of the water leaking out was at the connection point. This was not performed for experiment two since experiment two was conducted to support the conclusion of experiment one.
Possible errors for this experiment were the acetone bath which could account for the increase in mass for many of the caps. Even though the finished prints were allowed 15 minutes to dry, that could not have been long enough to account for the acetone saturation. To ensure that the acetone is completely dry, the prints were re-massed and retested after more than 24 hours had passed. It was found that after these 24 hours, the difference in masses for the caps was no different. The top of the cap was less malleable after all the acetone could dry. For future printing, it is encouraged to allow the finished part to dry for a least a couple of hours to make sure all acetone has evaporated.
Below are pictures that compare the results of different levels of acetone. This can be used a reference to show how increasing the amount, increased the layer of acetone that finished the part. The results on the outside show increasing smoothness and rounding at the edges. Internally, increasing the acetone resulted in a better seal. There was not a great difference between the 18 and 19 mL caps, which is why the interval of 14-19mL is the recommended interval for the acetone. The numbers on the cap indicate the amount of acetone used in the experiment.




Author: Merritt Allen
